A ferrofluid
is a liquid which becomes highly magnetized in the presence of a magnetic
field. The distinctive ‘spikey’ shape of a magnetized ferrofluid is caused by
the need to find the most stable shape in order to minimize the total energy of
the system, an effect known as the normal-field instability. The fluid is more
easily magnetized than the surrounding air, so is drawn out along the magnetic
field lines, resulting in the formation of peaks and troughs. However, the
extension of the ferrofluid is resisted by gravity and surface tension. The
formation of the corrugations lowers the magnetic energy of the system but
raises the gravitational energy and surface free energy. When these forces are
balanced, the minimum energy configuration is achieved. Because ferrofluids are
very easily magnetized (they have an incredibly high magnetic susceptibility),
the peaks can be produced using a small bar magnet.

Ferrofluids
are known as colloidal fluids and are composed of nanoscale ferromagnetic
particles suspended in a carrier fluid, usually water or an organic solvent
like kerosene, and coated with a surfactant to stop them clumping together in
the liquid. A typical composition would be 5% magnetic particles, 10%
surfactant and 85% carrier fluid.
The particles
in a ferrofluid have a diameter of 10 nanometers or less and are composed of a
ferromagnetic, highly magnetically susceptible compound such as magnetite (Fe
3O4)
or hematite (Fe
2O3). The particle size has to be small
enough to allow them to be evenly dispersed through the liquid by Brownian
motion (the random motion of particles in a liquid due to collisions which
other molecules) but large enough for them to each make a significant
contribution to the magnetic response of the fluid. Upon application of an
external magnetic field, the nanoparticles align with the field. However, once
the external field is turned off, the particles return to a random alignment. For
this reason, ferrofluids are classed as superparamagnets rather than
ferromagnets.
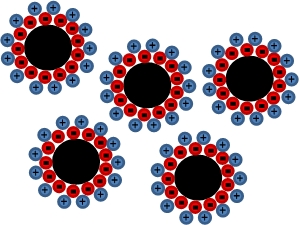
The surfactant's van der Waals forces stop the magnetic nanoparticles aggregating in the solution.
Different surfactants work in different ways but the general principle is that
the surfactant creates a layer around the particle which will repel other
coated nanoparticles. The diagram below illustrates the principles of an ionic
surfactant – the surfactant ions form a layer of charge around the
nanoparticle, repelling other charged, surfactant coated particles. Whilst the
addition of a surfactant is crucial, it has the negative effect of decreasing
the viscosity of the fluid in the magnetized state and making it ‘softer’. As
most applications require a ‘hard’ fluid in the magnetized form, this is an
important factor to consider when choosing the ferrofluid composition.
In 1963,
Steve Papell of NASA created ferrofluid for use as rocket fuel. His team of NASA
scientists were investigating methods of directing fluids in space and realized
that magnetic fluids could be completely controlled by the application and
variation of a magnetic field. The ferrofluid was mixed with liquid fuel and drawn
towards the ignition system with an external magnetic field. Ferrofluids have now
found use in many applications from small electronic devices to space crafts to
cancer treatments to art. In fact, ferrofluids are found in many common household
devices, including hard drives where they are used to seal the interior of the
device. When magnetized they form a barrier to dust and dirt which could damage
the delicate plates.
Ferrofluids
can have very high thermal conductivities and their heat transfer properties
are exploited in devices such as loud speakers where they are used to cool the
voice coil. In a loudspeaker, sound is produced when the voice coil vibrates
but this also generates unwanted heat. Ferrofluids lose their magnetism as they
are heated, fully losing their magnetic properties when heated to a high enough
temperature, known as the Curie temperature. If ferrofluid is placed around the
voice coil, a magnet placed near the coil will attract more cold ferrofluid
than hot ferrofluid because the colder ferrofluid will be more strongly
magnetized. This cold ferrofluid will absorb heat around the voice coil and
then be moved towards a heat sink as it is replaced by cooler ferrofluid.
Ferrofluids
are also the focus of current scientific research and have the potential to be
used in many medical applications. In magnetic drug targeting for example, where
drugs could be enclosed by ferrofluid and, once injected into the specific body
area requiring treatment, a magnetic field could be applied to keep the drugs
in this target area. The localization would limit exposure to the rest of the
body and enable the dosage level to be decreased, reducing the adverse side
effects experienced by the patient.